Procedure:
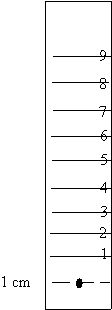
1. Cut four strips of filter paper at least 10 cm long.
2. Use a pencil to draw a line across the paper 1 cm from the bottom.
3. Use a pencil to label the paper as red, blue, green, black.
4. Make a
spot corresponding to the color on each of the papers, on the center of the
line.
5. From the center of the dot, measure up the center of the paper and
make a mark every centimeter.
6. Let the dot dry and darken the mark again.
7. Using the graduated cylinder, measure out 5 mL
of water, and pour into beaker.
8. Using the graduated cylinder, measure out 5 mL
of methanol and pour into the beaker.
9. Carefully stand all 4 paper strips in beaker, and crease edges to
keep them from falling
DO NOT ALLOW THE DOTS TO TOUCH THE LIQUID
10. Record observations until the solvent reaches
the edge of the beaker.
11. After the solvent has reached the edge of the beaker, remove the strips,
and put them on a piece of paper towel to dry. (CAUTION the dyes from the
marker can stain your hands and clothes. Do not touch the colored areas of the
paper.)
12.
Clean and dry beaker and graduated cylinder. Return all materials to where you
got them.
13.
Answer the questions.
14. Attach dried strips to sheet