Determining an Empirical
Lab Formula
Pre-Lab Discussion
In a sample of a compound, regardless of the size of the sample, the number of grams-atoms of one element in the sample divided by the number of gram- atoms of another element in the sample will form a small whole-number ratio. These small whole-number ratios can be used to determine the subscripts in the empirical formula of the compound. For example, suppose that in a 24-gram sample of z compound, there are 1.5 gram-atoms of carbon (18 of carbon) and 6 gram-atoms of hydrogen (6 g of hydrogen). These numbers form the small whole-number ratio of 1 to 4:
|
The 1-to-4 ratio means that for every 1 atom of carbon in the compound, there are d atoms of hydrogen. The empirical formula (if the compound is Ch4 (The compound's name is methane.)
In this experiment the number of gram-atoms of each of two elements in a binary compound will be experimentally determined. From this information, the empirical formula of the compound will be determined.
This experiment will help you understand better the concepts of gram atomic masses and empirical formulas
Purpose
Using mass relationships show that magnesium and oxygen combine in a definite whole-number ratio by mass.
Equipment
crucible and cover
scissors
iron ring
balance
clay triangle
safety goggles
crucible mugs
lab apron or coat dropper piper
Materials
Magnesium ribbon (Mg), 35 cm
Do not touch a hot crucible with your fingers, and be sure you use tongs to shift the position of the hot crucible cover in step 3. Use a hand to waft the gas given off in step 6 to your nose. Avoid directly inhaling reaction product gases. Do not pace any magnesium ribbon in an open flame.
Observe the caution alert symbols under "Procedure; and follow the precautions indicated. Tie back long hair and secure loose clothing when working with an open flame. Always wear safety goggles and a lab apron or coat when working in the lab
Procedure
1. Clean a crucible and cover. Dry them by heating them in the hottest part of a burner flame for 3 minutes. Allow them to cool. Measure the mass of just the crucible and record this as (a) under "Observations and Data."
2. Cut a 35-cm length of magnesium ribbon into 1 cm pieces. Place the pieces in the crucible and measure the mass of the crucible and its contents (b)
3. Cover the crucible and place it in a clay triangle (Figure 13-1). Heat gently for 2 minutes. Using crucible tongs, carefully tilt the cover to provide an opening for air to enter the crucible. Heat the partially covered crucible strongly for 10 minutes.
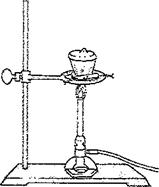
Figure 13-2
4. Turn off the bur burner cover the crucible, and allow the contents to cool. When the crucible is cool enough to touch, remove the cover and examine the contents. If any unreacted magnesium remains, replace the cover at a slight tilt, and reheat the crucible strongly for several minutes.
5. Put the cover all the way on and allow to cool. After making sure that all the magnesium has reacted, use a dropper pipet to add enough water to the crucible to just cover the contents. Wash any material that may have spattered onto the inside of the cover into the crucible.
6. Holding the burner in your hand, gently heat the contents of the uncovered crucible by moving the burner slowly back and forth. Avoid spattering. Observe the odor of the vapor given off by wafting it toward your nose. Record your observation as (d).
7. When all the liquid has boiled off repeat steps 5 and 6.
8. When all the liquid has boiled off a second time, strong y heat the uncovered crucible for v minutes.
9. Turn off the burner and allow the crucible and contents to cool. Measure the combined mass of the crucible + contents (c).
Observations and Data
a. Mass of empty crucible
b. Mass of crucible + Mg
c. Mass of crucible + oxide
d. Odor of vapor in step 6:
Calculations
1. Find the mass of magnesium used: b – a
2. Find the mass of oxygen that reacted: c - b
3. Find the number of g-atoms of Mg used:
g-atoms Mg = mass of Mg in g
24 g Mg/g-atom Mg
4. Find the number of g-atoms of O that reacted:
g-atoms Mg = mass of Mg in g
16 g O/g-atom O
5. Find the ratio of g-atoms of Mg to g-atoms of O:
Conclusions and Questions
1. Write the empirical formula of the oxide of magnesium based on your calculations from this experiment.
2. What is the ratio of the mass in grams of magnesium used to the mass in grams of oxygen that reacted? Relate this ratio to the law of definite proportions
3. Why is the ratio found in question 2 different from the ratio found in calculation 5 above?
4. In a chemical formula, explain the significance of subscripts in terms of atoms and molecules. In terms of gram-atoms and moles.
5. The molecular formula of hydrogen peroxide is H2O2 What is its empirical formula?
6. How is the chemical composition of carbon monoxide, CO, similar to that of carbon dioxide, CO2? How is it different?
7. A sample of sulfur having a mass of 1.28 g combines with oxygen to form a compound with a mass of 3.20 g. What is the empirical formula of the compound?