

Chapter 8 Review
1. The number of sulfur atoms in 96.3 g of sulfur is
a. 1 b. 3 c. 6 x 1023 d. 1.8 x 1024
2. The gram formula mass of aluminum sulfate, AL2(SO4)3, is
a. 342 g b. 246 g c. 150 g d. 123 g
3. A mole of oxygen gas (O2) is compared with a mole of lead (Pb). You can be certain that the sample of oxygen and the sample of lead have the same
a. volume b. mass c. number of particles d. all of the above
4. The empirical formula of a compound can be determined
a. if the masses of each element in a sample of the compound are known.
b. if the percentage composition of the compound is known
c. only if both “a” and “b” are known
d. if either “a” or “b” is known
5. Volumes of gases are usually stated under standard temperature and pressure conditions (STP). These conditions are
a. 0°C and 101.3 kPa
b. 0 K and 101.3
c. 25°C and 30 kPa
d. 25°C and 1000 kPa
6. A mole of hydrogen gas (H2) is the same amount as
a. one gram molecular mass of H2
b. one gram formula mass of H2.
c. two grams of H2.
d. all of the above
7. What is wrong with the figure below?
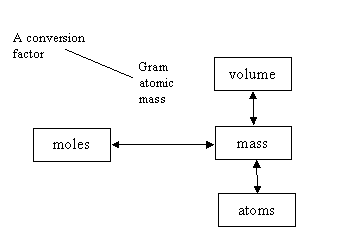
a. gram atomic mass is not a conversion factor
b. mass cannot be converted to moles
c. mass cannot be converted directly to volume
d. nothing is wrong with the figure
8.The number of particles is one gram atomic mass of any element is equal to
a. one dozen b. one hundred c. 2.5 x 1018 d. Avogadro’s number
9. If the atomic mass of element A is 5.0 times the atomic mass of element B, what amount of element A would have the same number of particles as 2.0 g of element B?
a. 0.40 g b. 1.00 g c. 2.50 g d. 10.0 g
10. When referring to the sum of the atomic masses of the atoms represented by the formula of a molecular substance the correct term to use is
a. atomic mass b. formula weight. c. molecular mass d. molecular weight
11. What is the mass of 0.0020 moles of NaOh?
12. How many atoms of sodium, Na, would be found in 100.0 g of Na?
13. What is the percentage composition of carbon monoxide, CO?
14. What is the percentage composition of a compound consisting of carbon and hydrogen if 58.0 g of the compound has only 10.0 g of hydrogen?”
15. Find the empirical formula of a compound if a 6.3-g sample is composed of 4.6 g of sodium and 1.6 g of oxygen.
16. What is the mass of 3.0 moles of fluorine atoms?
17. Describe the procedures you would follow to determine the percentage composition of a substance knowing the empirical formula of the substance.
18. How many grams of ammonia gas, NH3, are in a 44.8-dm3 sample measured at STP?
a. 17 g b. 34 g c. 6.02 x 1023 g d. 1.2 x 1024 g
19. The number of molecules of nitrogen, N2, found in 500.0 g of nitrogen gas is
a. 1 b. 5 c. 18 x 1023 d. 1 x 1025
20. What is the molecular formula of a compound that has a percentage composition of 58.0% sodium and 42.0% oxygen? The gram molecular mass of the compound is 78 g.
21. a. A compound decomposes to form 40.0 g of mercury and 2.2 dm3 of oxygen gas (O2) at STP. What is the empirical formula of the compound?
b. How many moles of SO2 are found in 100 dm3 of the compound?
22. The molar volume for all gases for STP is approximately the same. Provide a rationale for accepting an experimental result such as this.